- Company founded
- Preliminary establishment of core team
- Started the research and development of intelligent gynecology system project
- Completed the layout of five categories of products for clinical examination
- Completed GMP factory construction
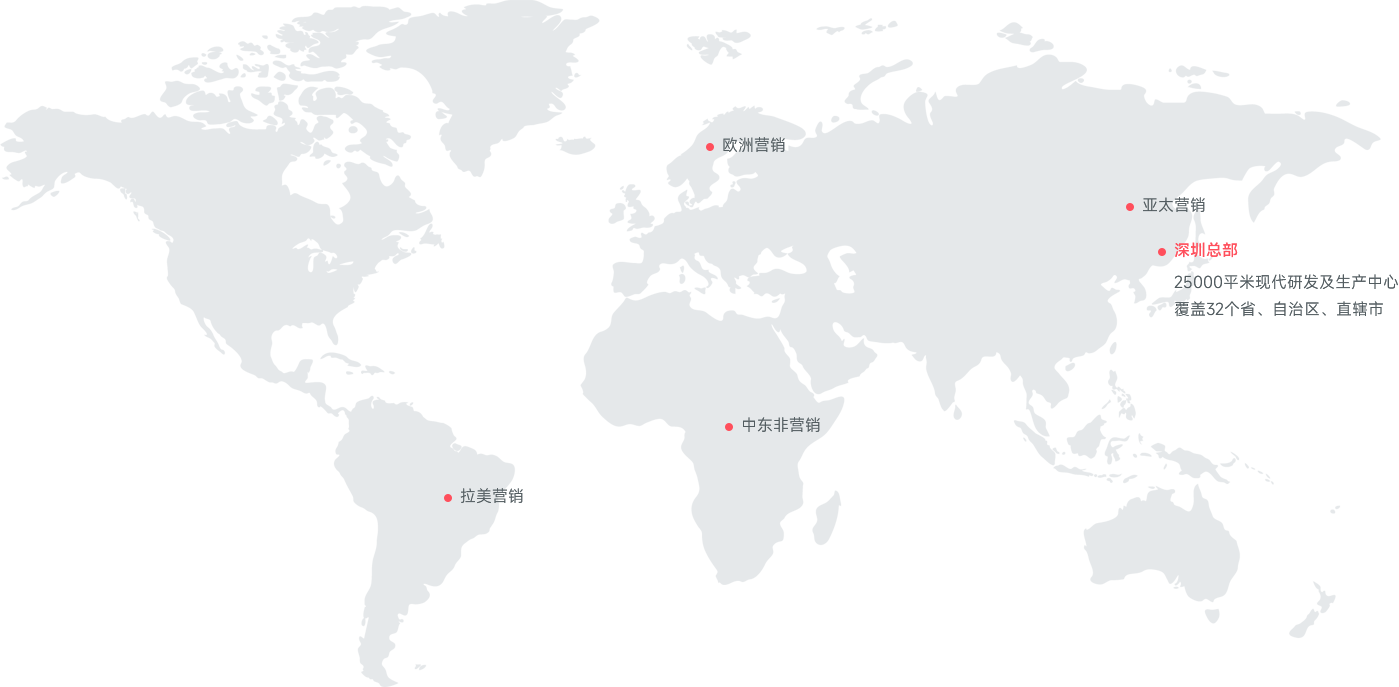
- 深圳总部






































 粤公网安备44030002003406
粤公网安备44030002003406